BioSenic focuses on bone pathologies and tissue repair for a return to normal function. Their common challenge: controlling inflammation.
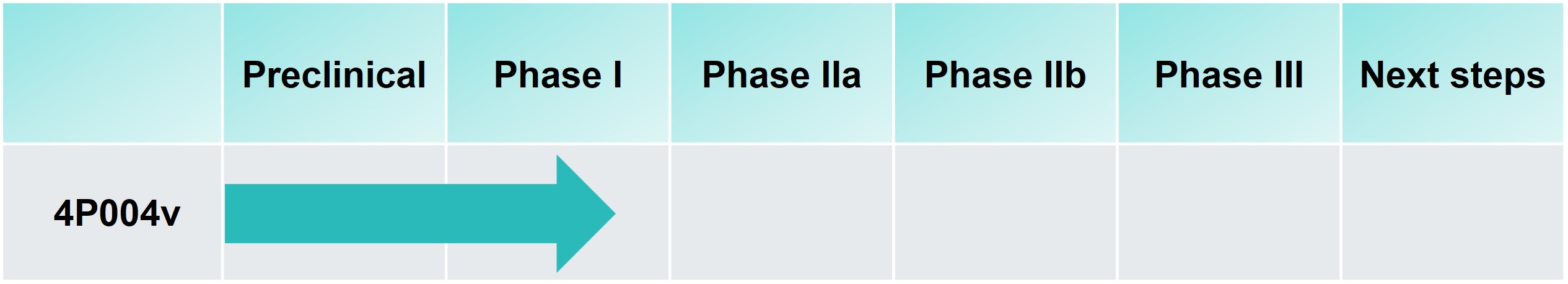
BioSenic is co-developing, with the French company 4Moving Biotech, 4P004v, a veterinary drug for dogs recovering from surgery after ligament repair. The aim is to modify the course of the disease for better post-operative recovery and long-term joint quality after cranial cruciate ligament (CCL) surgery. (CCL) surgery. Each year, more than 750,000 dogs undergo this type of surgery in the United States and Europe. With the drugs currently available on the market treating only the symptoms, 4P004v could meet a significant unmet need.
4p004v
With its unique anti-inflammatory, anti-catabolic, and regenerative action, 4P004v is positioned as the first-in-class candidate in veterinary orthopedics. 4P004 is a GLP-1 analog designed for intra-articular use in human knee osteoarthritis. The current objective is to demonstrate clinical proof of concept in dogs following ligament repair. The Phase I clinical study is ongoing.
- More
-
4Moving Biotech is a cutting-edge clinical-stage company pioneering research in osteoarthritis (OA). It is inspired by the work of renowned rheumatologist Professor Francis Berenbaum. Its unique human health product, 4P004, targets low-grade inflammation using GLP-1 analogues. This innovative application of GLP-1 analogues represents a promising avenue for natural treatment of the disease. By integrating advanced AI, real-world data, and innovative clinical trial design, 4Moving Biotech optimizes Phase II trial selection using data from 11.4 million patients.
Please note: 4P004v is a product currently under investigation and is not yet available for sale. This website is not intended to provide medical advice or replace care from healthcare professionals. Consult a doctor or healthcare provider for information about your diagnosis or condition.
ALLOB
Past development and discontinued.
ALLOB® is a differentiated allogeneic osteoblastic product with regenerative properties for the treatment of bone diseases. It is obtained from bone marrow stem cells cultured ex vivo. The term “allogeneic” means that the cells are taken from a healthy donor, as opposed to the “autologous” approach in which the cells are taken from the patient.
ALLOB® received orphan drug designation for osteonecrosis from the EMA in July 2013 and the FDA in January 2014. The product has also received orphan drug designation from the EMA and the FDA for osteogenesis imperfecta (brittle bone disease). ALLOB® has been classified as a tissue engineered product (non-combined) by the EMA under ATMP Regulation 1394/2007EC.
After validating two Phase I/IIA proof-of-concept clinical trials for the treatment of delayed-union fractures and spinal arthrodesis procedures, the latest results obtained in Phase IIb were unfortunately not conclusive enough.
JTA-004
Past and discontinued development.
JTA-004 is a new-generation, intra-articular injectable treatment for osteoarthritic knee pain. Developed by BioSenic, this unique patented blend of plasma proteins, hyaluronic acid—naturally present in the synovial fluid of all joints—and a fast-acting analgesic, JTA-004 aims to improve lubrication and protection of the cartilage in arthritic joints and relieve associated pain.
Unfortunately, the Phase III study of JTA-004 did not produce conclusive results overall.
However, post-hoc analysis demonstrated a positive effect on type 3 osteoarthritis, the most severe form of the disease accompanied by inflammation. BioSenic is considering selling this product if another company wishes to explore options for the future development of JTA-004 for this application.
Clinical Pipeline

test B




